Bio-catalysis has become a solution for sustainable and green medicine manufacturing due its safety and environmental impacts. Compared with traditional chemical process, Bio-catalysis has incomparable advantages in terms of chemo selectivity, efficiency and eco-friendliness.
AstaTech has been committed to “the research and development of green technologies for the healthy development of human and nature”. We have developed enzyme catalytic processes to break the patent barriers, including new enzyme discovery,screening,modification,fermentation and immobilization. We are also working with universities and enterprises for further business expansion and high-throughput screening.
We have involved bio-catalysis process to develop various products. The biocatalysis platform focuses on the layout of non-natural chiral amino acids, chiral amines, chiral alcohols and other products, and has successfully realized the development and production of key enzymatic catalytic processes in several series of products (enzyme resolution process, transaminase process, oxidoreductase process, lyase process, etc.). We are qualified to solve critical technical problems in the feasibility studies, R&D, process innovation and CDMO projects, etc.
The areas covered by AstaTech include:
The biocatalytic platform has established a commercial enzyme library, which has the ability to quickly screen enzymes with superior catalytic effects.
Enzyme type | Application of reactions | Example |
Hydrolase | Hydrolysis of esters, amides,nitriles and hydantoins | Lipases, proteases ,nitrile hydrolases, Hydantoinase, carbamoyl hydrolase, etc. |
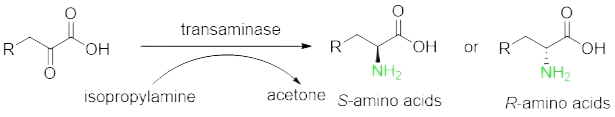
Transferase | Functional group transfer | Transaminase |
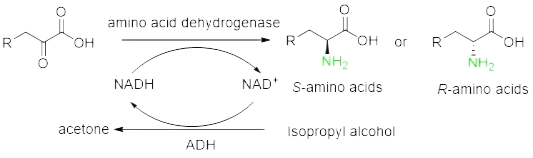
Oxidoreductase | Redox reaction | Ketoreductase , amino acid dehydrogenase , GDH(glucose dehydrogenase), FDH(formate dehydrogenase), etc. |
lyase | To form or eliminate form double bonds | Ammonia lyase |
aldolase | Condensation of functional groups | Aldolase |
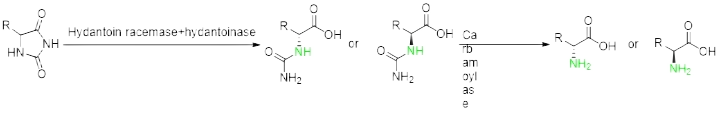
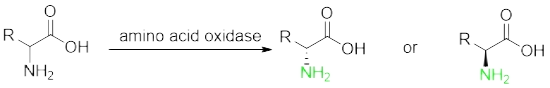
Prepare non-natural chiral amino acids through enzymatic resolution cyano Hydrolase, Transaminase, amino acid dehydrogenase, ammonia lyase, amino mutase and Fructose-bisphosphate aldolase.
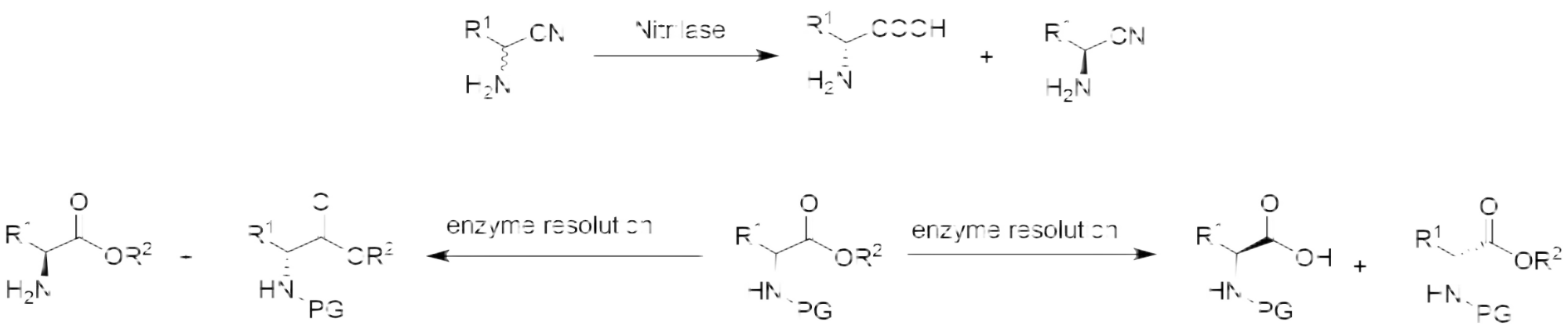
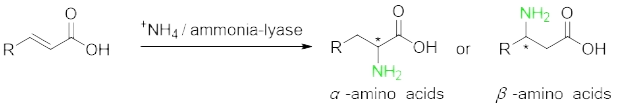

Chiral alcohol is a class of molecular blocks required for the synthesis of medicine. The preparation of a single isomer of alcohol by enzyme catalysis is in line with the development concept of green chemistry.

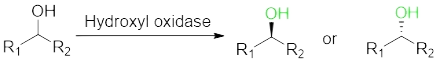
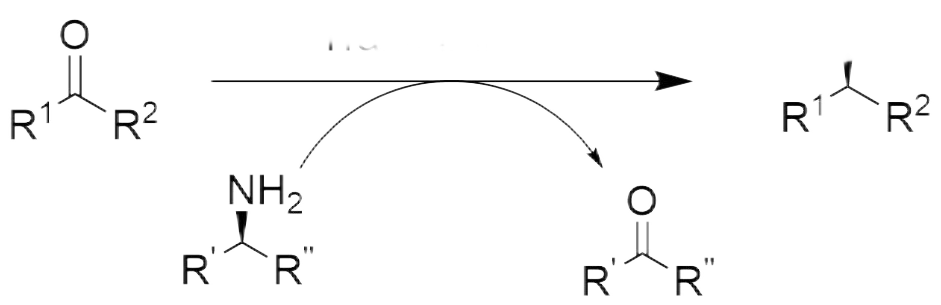
There are many types of chiral amine drug intermediates, accounting for a large proportion.Compared with the chemical amination reduction method, the efficiency of transaminase catalyzing the preparation of chiral amines from ketone substrates is higher.
Chiral amide is prepared through Hofmann rearrangement, which is often used in the synthesis of some statin drugs. The enzymatic separation route to synthesize chiral amide is more cost-effective.
WeChat
